OpsTrakker GMP Solutions
Contact us for help!
If you would like to discuss how your business can benefit from OpsTrakker solutions, please contact us.
If you would like to discuss how your business can benefit from OpsTrakker solutions, please contact us.

Countless plant operations are carried out using paper checklists and forms. These documents are oftentimes incomplete or executed improperly. Moreover, paper requires a physical presence for wet signatures and prohibits cross-team cooperation and remote work.
eForms are digitized forms which enable standardized activity completion and electronic data capture via tablet or web browser. OpsTrakker eForm solution has been created using a mobile-first approach to ensure they can be accessed and reviewed from anywhere.
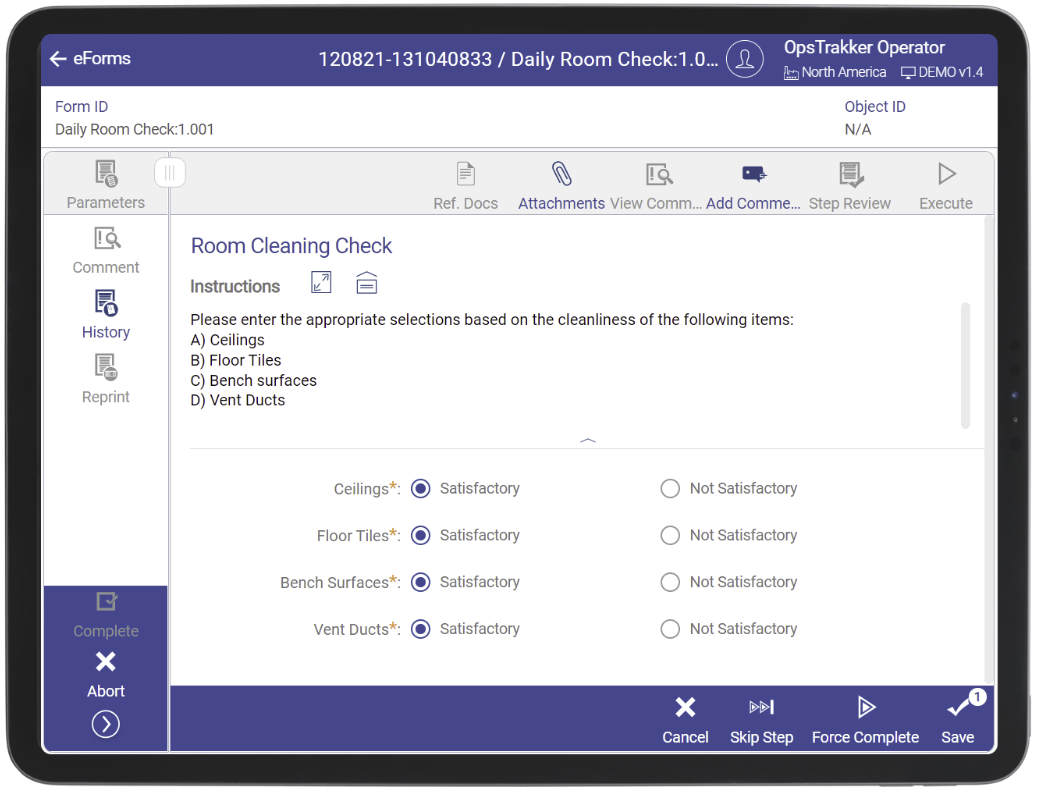
With electronic forms and checklists, OpsTrakker drives productivity and standardization so that all activities are complete and correct. Above all, digital forms and checklists eliminate paper based documents to help you achieve your digitalization and sustainability goals. OpsTrakker eForms have been created with GMP in mind. OpsTrakker is audit ready and compliant with federal regulations for electronic signatures (FDA 21 CFR Part 11 and EU Annex 11).
With more than 25 years of industry experience, we have assisted some of the biggest players in the industry advance their digitalization ambitions. Digitized forms and checklists not only make life easier for operational staff, but are also easier to audit and extract data. All information is stored in a central location and can be accessed quickly and easily by all authorized personnel without spending hours sifting through paper forms.
OpsTrakker eForms solution supports the range of operator activities for form entry. The workflow engine standardizes shop-floor activities and guides the user to complete activities right the first time. Our eForms are flexible and configurable to replace any paper-based GMP forms within your facilities.
The environmental impact of paper records is substantial. Each document must be controlled throughout its life cycle. By the end, the document must be archived and destroyed. Such a process can produce tens if not hundreds of thousands of GMP paper records across an organization. Taking such a view makes the environmental impact of such a system apparent. OpsTrakker eliminates the need for paper-based GMP records as all workflows and records are completed digitally. Thus, helping your organization reduce your environmental impact and support your sustainability goals.