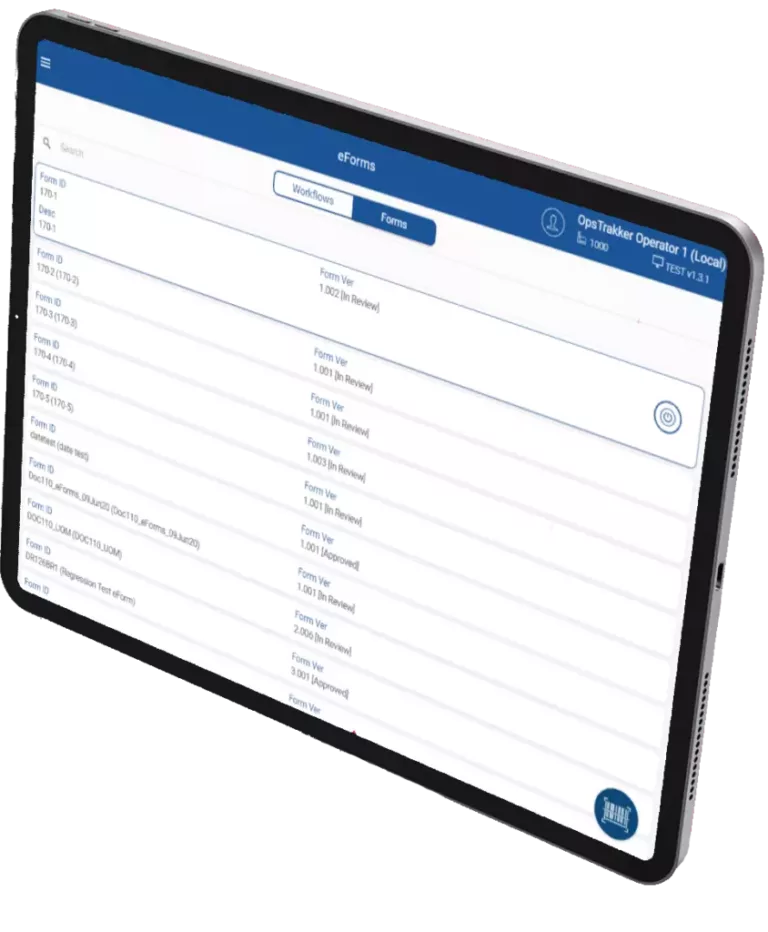
About OpsTrakker
Ensure right-first-time manufacturing and compliance with OpsTrakker
From paper on glass checklists to complex records (e.g. eLogbook, eBR), OpsTrakker provides high-quality and cost-effective digital solutions that replace paper-based records. Eliminate data input errors, reduce manufacturing review cycles and enhance your organization’s digitalization strategy with OpsTrakker.
Read More