GMP Compliant Digital Solution
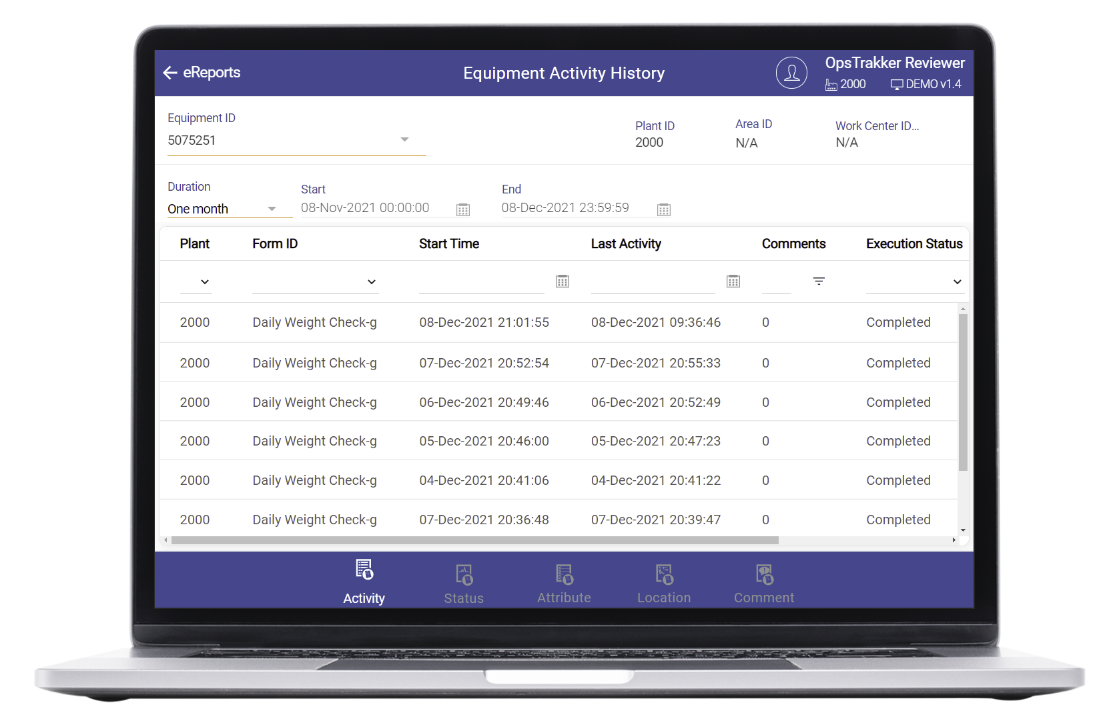
Review by Exception
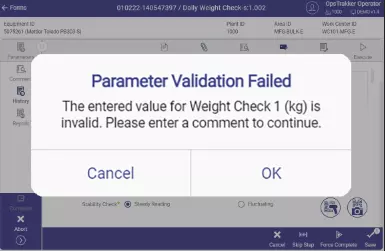
OpsTrakker enforces correct data entry, captures deviations with comments, and maintains the entire, proper, audit trail to achieve higher compliance levels
OpsTrakker’s electronic solutions facilitate compliant manufacturing operations for Pharmaceutical Manufacturing and the Life Sciences Spaces. Leveraging decades of experience implementing and validating digital systems, OpsTrakker facilitates compliance in a number of ways to ensure compliant operations with GMP regulations.
Both GMP and 21 CFR Part 11 compliant, OpsTrakker delivers off-the-shelf compliance functionality, to meet regulatory and organizational requirements:
- Off-the-Shelf Review by Exception
- In-House OpsTrakker Validation Support for Rapid Deployment
- Parameterization to maximize efficient form use and activity management
- Delivery of full vendor qualification package
- Complete Audit Trail for all internal and external transactions
- Activity guidance upon form or workflow execution
- And More...
Available for iOS, Android, and supported browsers, advance your digitalization strategy with OpsTrakker.