Request a Demo
Discover OpsTrakker eLogBook
Learn why leading Life Science companies use OpsTrakker to digitize GxP paper records
eLogbook Solution
Enable Real-Time Equipment Use and Status Management
After many years implementing other software and hearing countless feedback from the customer, we saw the short comings and benefits of industry leaders at the time. We decided to take the things we liked and didn’t like and develop our own software. OpsTrakker eLogBook was designed to solve the customers problems and deliver a bundle of benefits in a timely and cost-effective fashion.
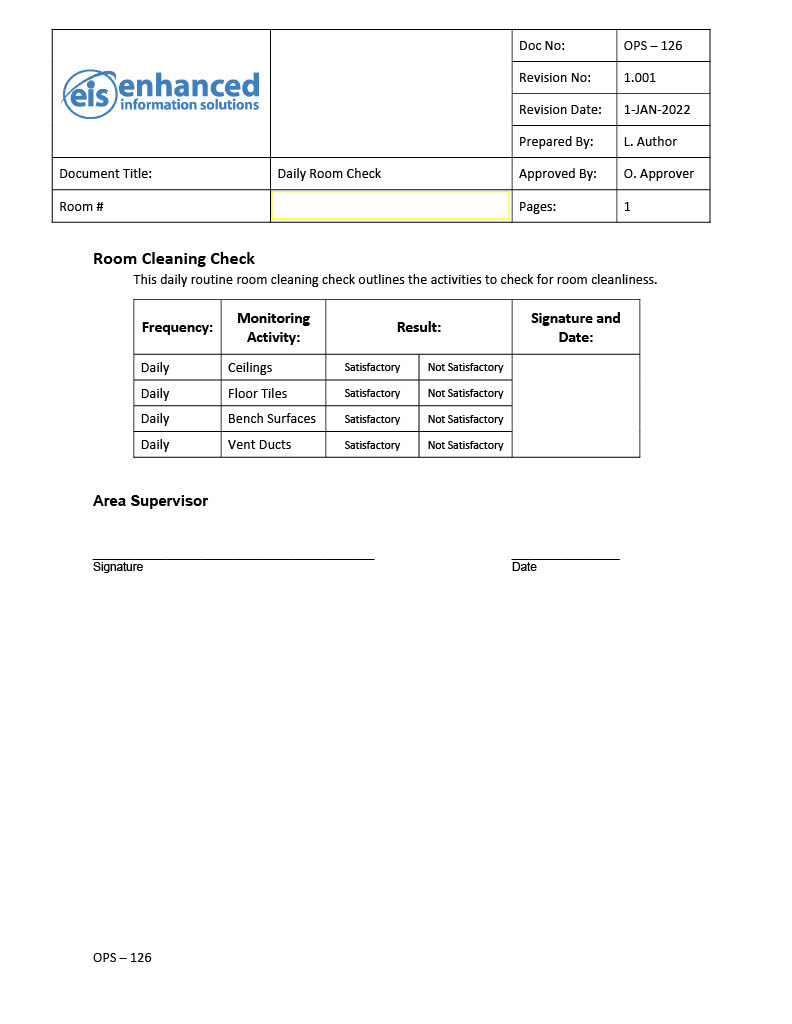 OpsTrakker’s eLogBook solution is specifically designed to eliminate paper use-logs and logbooks in GMP manufacturing facilities, to enhance manufacturing capabilities. With 25+ years in the industry, our knowledge and expertise has allowed us to create an digital logbook solution that is easy to implement, user-friendly and compliant with federal regulations. OpsTrakker eLogBook enables highly configurable records for all equipment types and activities, including usage, cleaning, calibration, maintenance, and more.
OpsTrakker’s eLogBook solution is specifically designed to eliminate paper use-logs and logbooks in GMP manufacturing facilities, to enhance manufacturing capabilities. With 25+ years in the industry, our knowledge and expertise has allowed us to create an digital logbook solution that is easy to implement, user-friendly and compliant with federal regulations. OpsTrakker eLogBook enables highly configurable records for all equipment types and activities, including usage, cleaning, calibration, maintenance, and more. Problems Solved
 OpsTrakker’s eLogBook solution is specifically designed to eliminate paper use-logs and logbooks in GMP manufacturing facilities, to enhance manufacturing capabilities. With 25+ years in the industry, our knowledge and expertise has allowed us to create an digital logbook solution that is easy to implement, user-friendly and compliant with federal regulations. OpsTrakker eLogBook enables highly configurable records for all equipment types and activities, including usage, cleaning, calibration, maintenance, and more.
OpsTrakker’s eLogBook solution is specifically designed to eliminate paper use-logs and logbooks in GMP manufacturing facilities, to enhance manufacturing capabilities. With 25+ years in the industry, our knowledge and expertise has allowed us to create an digital logbook solution that is easy to implement, user-friendly and compliant with federal regulations. OpsTrakker eLogBook enables highly configurable records for all equipment types and activities, including usage, cleaning, calibration, maintenance, and more. - Inaccurate data input
- Misplaced physical logbooks
- Illegible completion of use-logs
- Incorrect calculations
- High cost storing paper logbooks
- Inability to analyze data
- Relabeling throughout production
Solution Features
- FDA 21 CFR Part 11 compliant
- Biometric Authentication
- Form Parameterization
- Real-time status & attributes
- Barcode & QR Code Entry
- Activity completion enforcement
- Integration with ERP & historians
Benefits Delivered
 OpsTrakker’s eLogBook solution is specifically designed to eliminate paper use-logs and logbooks in GMP manufacturing facilities, to enhance manufacturing capabilities. With 25+ years in the industry, our knowledge and expertise has allowed us to create an digital logbook solution that is easy to implement, user-friendly and compliant with federal regulations. OpsTrakker eLogBook enables highly configurable records for all equipment types and activities, including usage, cleaning, calibration, maintenance, and more.
OpsTrakker’s eLogBook solution is specifically designed to eliminate paper use-logs and logbooks in GMP manufacturing facilities, to enhance manufacturing capabilities. With 25+ years in the industry, our knowledge and expertise has allowed us to create an digital logbook solution that is easy to implement, user-friendly and compliant with federal regulations. OpsTrakker eLogBook enables highly configurable records for all equipment types and activities, including usage, cleaning, calibration, maintenance, and more. - Improved operator efficiency
- Support for iOS, Android, and PCs
- Equipment data capture
- Review by Exception
- Eliminate Paper headaches
- Right first time execution
- Audit-ready environment
Eliminate Paper and Go Digital

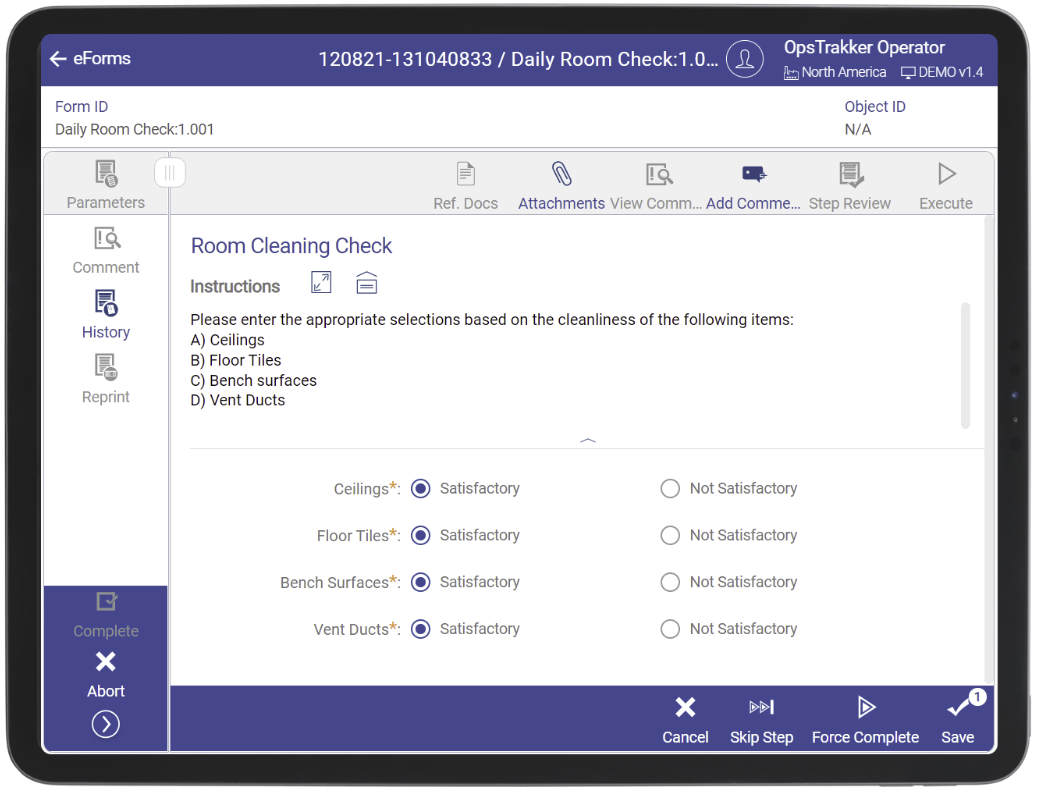
(Slide to Reveal)
OpsTrakker’s eLogBook solution is specifically engineered to eliminate paper use-logs and logbooks in GMP manufacturing facilities and enhance manufacturing capabilities. With 30+ years in the industry, our knowledge and expertise has allowed us to create a digital logbook solution that is easy to implement, user-friendly and compliant with federal regulations. OpsTrakker eLogBook enables highly configurable records for all equipment types and activities, including usage, cleaning, calibration, maintenance, and more.
 OpsTrakker’s eLogBook solution is specifically designed to eliminate paper use-logs and logbooks in GMP manufacturing facilities, to enhance manufacturing capabilities. With 25+ years in the industry, our knowledge and expertise has allowed us to create an digital logbook solution that is easy to implement, user-friendly and compliant with federal regulations. OpsTrakker eLogBook enables highly configurable records for all equipment types and activities, including usage, cleaning, calibration, maintenance, and more.
OpsTrakker’s eLogBook solution is specifically designed to eliminate paper use-logs and logbooks in GMP manufacturing facilities, to enhance manufacturing capabilities. With 25+ years in the industry, our knowledge and expertise has allowed us to create an digital logbook solution that is easy to implement, user-friendly and compliant with federal regulations. OpsTrakker eLogBook enables highly configurable records for all equipment types and activities, including usage, cleaning, calibration, maintenance, and more. Join the Industry Leaders using OpsTrakker


